Inorganic, Energy and Materials Chemistry
- B.S. Centenary College of Louisiana (1967)
- Ph.D. Indiana University (1971)
- Postdoctoral Fellow, University of Virginia (1971-73)
- Postdoctoral Fellow, Massachusetts Institute of Technology (1973-74)
Our research in inorganic chemistry, energy-storage and materials science includes synthetic studies in main-group, transition metal and materials chemistry along with physical and structural investigations of molecular, polymeric, and solid-state materials. Brief overviews of ongoing projects are presented in the following sections.
Alternative Energy Carriers: New Chemical Methods for Hydrogen Storage
The development of efficient methods for hydrogen storage is a major hurdle that must be overcome to enable the use of hydrogen as an alternative energy carrier. We are exploring the use of chemical hydrides such as ammonia borane and ammonia triborane to store and deliver large amounts of hydrogen through dehydrogenation and/or hydrolysis reactions. As depicted in the example in Figure 1, we have shown that the rate and the extent of hydrogen release from these amineboranes can be significantly increased through the use of metal catalysts, ionic liquids and/or chemical additives. We are continuing to investigate both new methods for the controlled hydrogen release from amineboranes and the development of energy-efficient methods for their regeneration from spent-fuel products.
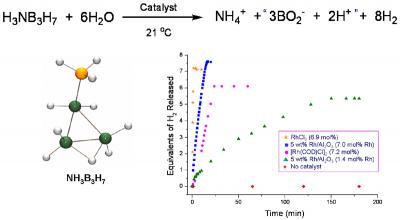
Figure 1. Rhodium catalyzed hydrolysis of ammonia triborane
Chemical Precursors to Ultra High Temperature Aerospace Materials
The production of complex structural and electronic materials in useable forms is one of the most challenging problems of modern solid-state chemistry and materials science. Our research in this area is focused on the design, syntheses and applications of new processible molecular and/or polymeric precursors to advanced carbide, nitride and boride ceramics that allow the formation of these technologically important materials in forms that cannot be produced with conventional methods. We are especially interested in ultra high temperature materials, such as HfB2 and ZrB2 based composites, that are potentially important in hypersonic (i.e. flying faster than 5 times the speed of sound) aerospace vehicles (Figure 2). A second part of the project is focused on the formation and properties of micro- and nanostructured ceramics, including fibers, tubes and porous materials.
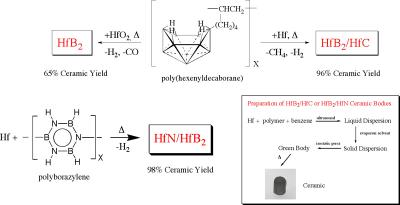
Figure 2. New chemical precursor systems for ultra high temperature hafnium-ceramics that were developed at Penn
Transition Metal-Promoted Reactions of Inorganic Compounds
We are developing new general, metal-catalyzed methodologies that enable the systematic, high-yield syntheses of important polyborane compounds and materials. Our goals are both to discover new types of catalytic reactions and to develop an understanding of their fundamental reaction mechanisms and controlling factors.
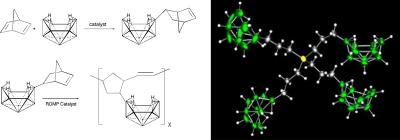
Figure 3. (Left) Metal-catalyzed synthesis of the poly(norbornenyldecaborane) polymer; and (Right) The crystallographically determined structure of a new dendritic decaborane (you can manipulate this molecule online at
Ionic Liquid Promoted Reactions
Ionic liquids have properties that make them attractive solvents for synthesis, including: negligible vapor pressures, thermal stability to elevated temperatures; the ability to dissolve a range of compounds, salts and gases, immiscibility with many hydrocarbons and/or water thus enabling two-phase reaction systems, weakly-coordinating anions and cations that provide a polar, inert reaction medium, and the ability to stabilize polar intermediates and/or transition states. While ionic liquids have been widely employed for organic synthesis, our recent work showing that decaborane olefin-hydroboration and alkyne-insertion reactions proceed in biphasic ionic-liquid/hydrocarbon solvents without the need of the catalysts required in conventional solvents, was the first demonstration of the unique activating effects of ionic liquids for polyborane syntheses. We are continuing to explore the scope of ionic liquid mediated polyborane reactions along with experimental and computational studies of the mechanisms by which these reactions occur.
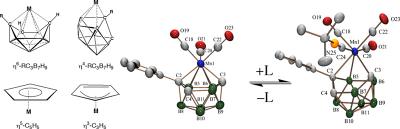
Inorganometallic Chemistry
Because of the unusual ranges of their accessible charges and coordination geometries, polyboranes can function as versatile ligands that can stabilize transition metals in a much wider array of environments than their organic counterparts, such as the cyclopentadienide anion. We are using integrated synthetic, structural (NMR and X-ray crystallography), electrochemical, and computational (DFT/GIAO) investigations to elucidate the nature of polyborane-metal bonding. The unique properties of metallapolyborane complexes are also being exploited to design new metallocene-like complexes with chemical, optical and/or bioactivity properties of importance to solid-state and/or anticancer applications.