Physical Chemistry, Molecular Structure and Dynamics
- B.A. Douglass College, Rutgers University (1976)
- Ph.D. Columbia University (1981)
- NSF Postdoctoral Fellow, AT&T Bell Laboratories (1981-82)
Honors and Awards
- Herbert P. Broida Prize, American Physical Society (2019)
- Philadelphia American Chemical Society Section Award (2018)
- Editor-in-Chief, The Journal of Chemical Physics (2009-2018)
- Member, National Academy of Sciences (2016)
- Francis P. Garvan-John M. Olin Medal, American Chemical Society (2014)
- Fellow, American Academy of Arts and Sciences (2008)
- Bourke Lectureship, Faraday Division of the Royal Society of Chemistry (2005)
- Visiting Miller Research Professor, Berkeley (2003)
- Guggenheim Fellowship (2002-03)
- Fellow of the American Physical Society (1993), the American Association for the Advancement of Science (1997), and the American Chemical Society (2010)
- Alfred P. Sloan Research Fellow (1987)
- Camille and Henry Dreyfus Young Faculty Award (1982), Teacher-Scholar Award (1986)
Criegee intermediates: Research in the Lester laboratory is currently focused on the photo-induced chemistry of Criegee intermediates. Alkene ozonolysis is a primary oxidation pathway for alkenes emitted into the troposphere and an important mechanism for generation of atmospheric OH radicals, particularly in low light conditions, urban environments, and heavily forested areas. Alkene ozonolysis proceeds through Criegee intermediates, R1R2COO, which eluded detection until very recently. In the laboratory, the simplest Criegee intermediate, CH2OO, and methyl-substituted Criegee intermediates, CH3CHOO and (CH3)2COO, have now been generated by an alternative synthetic route and detected by VUV photoionization. This laboratory has further shown that UV excitation of the Criegee intermediates on a strong π*←π transition induces photochemistry, which involves multiple coupled excited state potentials and yields both O3P and O1D products. This group has also demonstrated that IR excitation of methyl-substituted Criegee intermediates in the CH stretch overtone region initiates unimolecular decay. The latter enables direct examination of the hydrogen transfer reaction leading to OH products, which is a key non-photolytic source of OH radicals in the atmosphere.
Hydrogen trioxide radical: This laboratory obtained the first infrared spectrum of the hydrogen trioxide (HOOO) radical, an intermediate invoked in the H + O3 and O + HO2 atmospheric reactions as well as the efficient vibrational relaxation of OH radicals by O2. There had been much debate in the literature as to whether HOOO is stable or metastable with respect to the OH + O2 limit, as well as the relative stability of the cis and trans conformers. We have characterized the geometric structure, vibrational frequencies, and stability of the cis and trans conformers of HOOO and its deuterated analog. In particular, by measuring the OH product state distribution following IR excitation of HOOO, we have directly determined the stability of trans-HOOO and shown that is much greater than prior estimates. As a result, HOOO may act as temporary sink for OH radicals and be present in measurable concentrations in the Earth's atmosphere. The experimental stability indicates that 25% of the OH radicals in the vicinity of the tropopause may be bound to O2, rather than free OH radicals. Studies of combination bands in the fundamental OH stretch region reveal nearly all other vibrational modes of trans- and cis-HOOO. We have subsequently derived a torsional potential from our spectroscopic data to obtain the relative stability of the cis and trans conformers and isomerization barrier, which are critical for atmospheric modeling of HOOO.
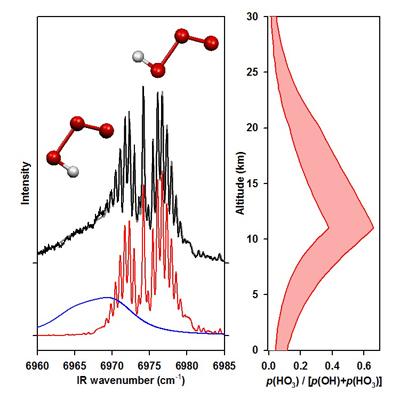
IR action spectrum of cis- and trans-HOOO in the OH overtone region (left), and fraction of atmospheric OH predicted to exist as HOOO (right).
Dynamical signatures of quenching: Collisional quenching of electronically excited OH A2Σ+ radicals has been extensively investigated because of its impact on OH concentration measurements in atmospheric and combustion environments. Yet little is known about the outcome of these events, except that they facilitate the efficient removal of OH population from the excited A2Σ+ electronic state by introducing nonradiative decay pathways. The quenching of OH A2Σ+ by H2 and D2 has emerged as a benchmark system for studying the nonadiabatic processes that lead to quenching. Theoretical calculations indicate that a conical intersection funnels population from the excited to ground electronic surfaces. Our studies examined the Doppler profiles for the H/D-atom products of reactive quenching, which show that most of the excess energy results in vibrational excitation of ‘hot’ water products. Our work also focused on characterizing the nonreactive quenching process, where OH X2Π products are generated with a remarkably high degree of rotational excitation and lambda-doublet specificity. The OH quantum state distribution directly reflects the anisotropy and A′ symmetry of the conical intersection region. We also demonstrated for H2 and D2 collision partners that reaction accounts for nearly 90% of the quenched products. These distinctive dynamical signatures of passage through a conical intersection region have sparked intense theoretical interest in this system.
We gratefully acknowledge financial support from the National Science Foundation under Grant No. NSF CHE-1362835 and the Chemical Sciences, Geosciences and Biosciences Division, Office of Basic Energy Sciences, Office of Science, U.S. Department of Energy under Grant No. DE-FG02-87ER13792.
A. S. Hansen, T. Bhagde, K. B. Moore III, D. R. Moberg, A. W. Jasper, Y. Georgievskii, M. F. Vansco, S. J. Klippenstein, and M. I. Lester, “Watching a Hydroperoxyalkyl Radical (•QOOH) Dissociate”, Science 373, 679-682 (2021)
M. F. Vansco, B. Marchetti, and M. I. Lester, “Electronic spectroscopy of methyl vinyl ketone oxide: A four-carbon unsaturated Criegee intermediate from isoprene ozonolysis”, J. Chem. Phys. 149, 244309 (2018).
V. P. Barber, S. Pandit, A. M. Green, N. Trongsiriwat, P. J. Walsh, S. R. Klippenstein, and M. I. Lester, “Four carbon Criegee intermediate from isoprene ozonolysis: Methyl vinyl ketone oxide synthesis, infrared spectrum, and OH production”, J. Am. Chem. Soc. 140, 10866–10880 (2018).
M. I. Lester and S. J. Klippenstein, “Unimolecular decay of Criegee intermediates to OH radical products: Prompt and thermal decay processes”, Acc. Chem. Res. 51, 978-985 (2018).
Y. Fang, F. Liu, V. P. Barber, S. J. Klippenstein, A. B. McCoy, and M. I. Lester, “Communication: Real time observation of unimolecular decay of Criegee intermediates to OH radical products”, J. Chem. Phys. 144, 061101 (2016).
N. M. Kidwell, H. Li, X. Wang, J. M. Bowman, and M. I. Lester, “Unimolecular dissociation dynamics of vibrationally activated CH3CHOO Criegee intermediates to OH radical products”, Nat. Chem., 8, 509-14 (2016).
H. Li, Y. Fang, N. M. Kidwell, J. M. Beames, and M. I. Lester, “UV photodissociation dynamics of the CH3CHOO Criegee intermediate: Action spectroscopy and velocity map imaging of O-atom products”, J. Phys. Chem. A. 119, 8328-37 (2015).
F. Liu, J. M. Beames, A. S. Petit, A. B. McCoy, and M. I. Lester, “Infrared-driven unimolecular reaction of CH3CHOO Criegee intermediates to OH radical products”, Science 345, 1596-1598 (2014).
J. H. Lehman and M. I. Lester, “Dynamical outcomes of quenching: Reflections on a conical intersection”, Ann. Rev. Phys. Chem. 65, 537-55 (2014).
J. H. Lehman, H. Li, J. M. Beames and M. I. Lester, “Communication: Ultraviolet photodissociation dynamics of the simplest Criegee intermediate CH2OO”, J. Chem. Phys. 139, 141103 (2013).
J. M. Beames, F. Liu, L. Lu, and M. I. Lester, “Ultraviolet spectrum and photochemistry of the simplest Criegee intermediate CH2OO”, J. Am. Chem. Soc. (Communication) 134, 20045-48 (2012).
J. H. Lehman, M. I. Lester, and D. H. Yarkony, “Reactive quenching of OH A2Σ+ by O2 and CO: Experimental and nonadiabatic theoretical studies of H- and O-atom product channels”, J. Chem. Phys. 137, 094312 (2012).
J. M. Beames, F. Liu, M. I. Lester, and C. Murray, “Communication: A new spectroscopic window on hydroxyl radicals using UV+VUV resonant ionization”, J. Chem. Phys. 134, 241102 (2011).
J. M. Beames, M. I. Lester, C. Murray, M. E. Varner, and J. F. Stanton, “Analysis of the HOOO Torsional Potential”, J. Chem. Phys. 134, 044304 (2011).
C. Murray, E. L. Derro, T. D. Sechler, and M. I. Lester, “Weakly bound molecules in the atmosphere – a case study of HOOO”, Acc. Chem. Res. 42, 419-427 (2009).
E. L. Derro, T. D. Sechler, C. Murray, and M. I. Lester, “Observation of combination bands of the HOOO and DOOO radicals using infrared action spectroscopy”, J. Chem. Phys. 128, 244313 (2008).
B. A. O’Donnell, E. X. J. Li, M. I. Lester, and J. S. Francisco, “Spectroscopic identification and stability of the intermediate in the OH + HONO2 reaction”, Proc. Natl. Acad. Sci. 105, 12678-12683 (2008).
I. M. Konen, I. B. Pollack, E. X. J. Li, M. I. Lester, M. E. Varner, and J. F. Stanton, "Infrared overtone spectroscopy and unimolecular decay dynamics of peroxynitrous acid", J. Chem. Phys. 122, 094320 (2005).

